Abstract
Introduction: Classical Hodgkin lymphoma (HL) is a malignancy of mature (post germinal center) B-lymphocytes that afflicts almost 20,000 individuals annually in North America and Europe. While the majority of patients diagnosed with HL are cured with multi-agent chemotherapy, 15 % of patients are refractory to conventional chemotherapy and almost half of patients with high-risk disease relapse. For these patients few effective therapeutic options exist. Only three drugs have been approved in the last 30 years for relapsed HL and thus additional novel therapies are needed. Bruton tyrosine kinase (BTK) is a member of the TEC family and plays a central role in B-cell signaling, activation, proliferation and differentiation. Inhibition of BTK with ibrutinib in cell lines and animal models demonstrates activity against HL suggesting potential therapeutic benefit in patients with relapsed or refractory HL. Here we describe preliminary safety and efficacy analysis of the first 6 evaluable patients treated in this phase II trial.
Methods: Patients >18 years with histologically confirmed classical HL who were ineligible for or had disease relapse after an autologous stem cell transplant were eligible. In addition all patients must have had prior exposure to brentuximab vedotin. Patients were treated with ibrutinib 560 mg orally daily until progression or unacceptable toxicity. The primary objective was overall response rate (ORR) as assessed by 2007 IWG criteria with secondary endpoints including safety, duration of response (DOR) and progression free survival (PFS).
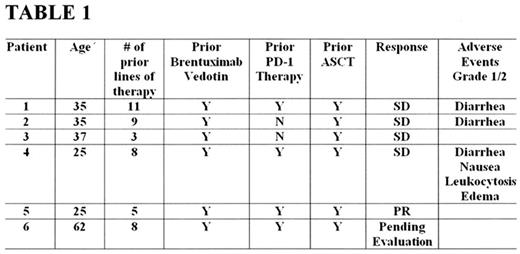
Results: To date 9 patients have been enrolled with 6 evaluable patients for analysis. (see table 1). Median age was 36 (range 25-62). Median number of prior therapies was 8 (range 3-11). All patients had prior brentuximab vedotin, and had prior autologous stem cell transplant. Four of six patients (67%) patients have received prior anti-PD-1 therapy. With a median follow up of 10 months the ORR is 17% with 1 patient demonstrating partial remission (PR) and 4 patients with SD. The median time to response was 3.2 weeks with a median duration of response (DOR) of 3.5 months. All adverse events (AE) were grade 1/2 and the most common AEs related to therapy included diarrhea in 3 patients, edema in 1 patient, nausea in 1 patient and leukocytosis in 1 patient. There were no grade 3, 4, or 5 adverse events noted.
Conclusion: Ibrutinib is well tolerated in patients with relapsed/ refractory HL with adverse events consistent with those seen in other lymphoma histologies treated with ibrutinib monotherapy. Early results of efficacy suggest tumor activity in a heavily pretreated population with an ongoing PR, however further follow up is needed. These results support continued assessment of the role of BTK inhibition with ibrutinib in Hodgkin lymphoma.
Ramchandren: Pharmacyclics: Research Funding; MERCK: Research Funding; Seattle Genetics: Consultancy; Janssen: Research Funding. Phillips: KITE: Consultancy; Seattle Genetics: Consultancy; Pharmacyclics: Consultancy; Incyte: Other: Travel/Expenses. Pregja: Pharmacyclics: Other: Data Safety Monitoring Committee ; Prothena: Consultancy; Celgene: Consultancy, Research Funding; Janssen: Consultancy; BMS: Consultancy, Research Funding; Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Aghamohammadi: Gillead: Speakers Bureau; Gilead: Speakers Bureau. Wesson: seattle genetics: Honoraria. Fanale: SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MOLECULAR TEMPLATES: Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GENENTECH: Research Funding; TAKEDA: Honoraria, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TAKEDA: Honoraria, Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC THERAPEUTICS: Research Funding; MOLECULAR TEMPLATES: Research Funding; ONYX: Research Funding; ADC THERAPEUTICS: Research Funding; ONYX: Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees; ONYX: Research Funding; TAKEDA: Honoraria, Research Funding; MOLECULAR TEMPLATES: Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GENENTECH: Research Funding; GENENTECH: Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal